
Zwitterions are functional in the medicinal chemistry design considerations when working with acid, basic or neutral leads. It is due to the fact that the positive and negative signs on the amine oxide denote formal charges. Generally, dipolar compounds are not considered as zwitterions. Zwitterions are referred to as “inner salts” sometimes. To prevent subaquatic organisms from building up on boats and piers in the marine industry, Zwitterionic polymers are used. Some of the most popular uses of zwitterions include medical implants, drug delivery, blood contact sensor, separation membrane, and antifouling coatings of biomedical implants (which help to prevent the build-up of microbial adhesion and biofilm formation). It has the potential to be used in a wide range of medical and biological-related fields. Zwitterions are widely used in the process of separating protein molecules through the SDS PAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis) method, which is one of the most popular techniques used in the field of molecular biology. K a2 = the equilibrium constant of the base. K a1 = the equilibrium constant of the acid. The pH value at the isoelectric point is calculated from the equilibrium constants such as acid and base of the Zwitterion. The solubility of a molecule at a given pH is also impacted by the pI value. In amino acids, the amino group is an effective proton acceptor and the carboxyl group is an effective proton donor. In this case, molecules become more charged either positively or negatively as a result of gain or loss in the number of protons. Generally, the pH has a great impact on the net charge on a molecule and its surrounding environment.
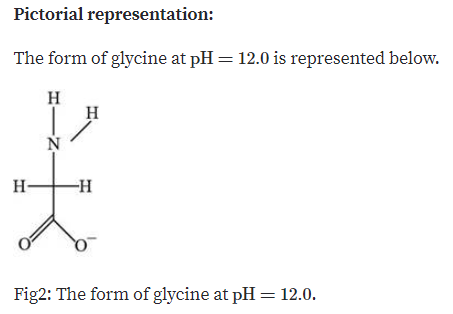
One of the main properties of a Zwitterion is that it has an isoelectric point, which is represented by pI, pH(I), IEP. These compounds contain quaternary ammonium cations. The atoms have stable, separated unit electrical charges in Zwitterrion Compounds.
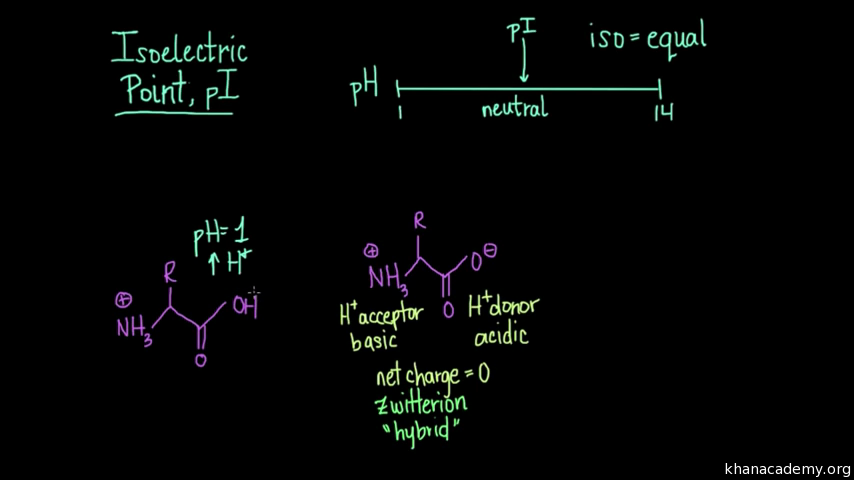
In this type of ion, the charged atoms are generally held together by one or more covalent bonds. It can be formed by compounds that have both acid and base groups in their molecules such as ampholytes. The following are some of the characteristics of Zwitterion: This is because the zwitterion changes to an anion in the presence of sufficient alkaline solution, and zwitterion changes to a cation in the presence of sufficient acid solution. To evaluate whether a substance is zwitterionic or not, the pH range must be specified. The existence of amino acids as dipolar ions in a solid state is called zwitterions. Therefore, zwitterions are electrically neutral as the net formal charge is usually zero.Ī zwitterion is a molecule that includes both positive and negative regions of charge. A zwitterion is an ion that has both positive and negative electrical charges. The term ‘Zwitterion’ is derived from the German word ‘zwitter’, which means ‘hybrid’ or ‘hermaphrodite’.


 0 kommentar(er)
0 kommentar(er)
